 General Rules of
Solubility:
General Rules of
Solubility: General Rules of
Solubility:
General Rules of
Solubility: |
Any substance that can form 0.1 M or more concentrated is soluble. |
 |
Any substance that fails to reach 0.1 M is defined to be insoluble. |
 |
VERY FEW substances have their maximum solubility near to 0.1 M. |
 |
Almost every substance of any
importance in chemistry is either much MORE soluble or much LESS
soluble. |
| Formula |
Crystal System |
Cell Dimensions |
Solubility |
|||
|---|---|---|---|---|---|---|
| a |
b |
c |
H2O |
H2SO4 |
||
| PbSO4 |
Orthorhombic Dipyramidal |
8.48 |
5.398 | 6.958 | 0.0425 |
Less soluble |
| a:b:c =1.5709:1:1.2889 | Ksp=1.96E-08 | |||||
| BaSO4 |
Orthorhombic Dipyramidal |
8.878 | 5.45 | 7.152 | 0.0022 |
Less soluble |
| a:b:c =1.6289:1:1.3122 | Ksp=8.89E-11 | |||||
| SrSO4 |
Orthorhombic Dipyramidal |
8.359 | 5.352 | 6.866 | 0.113 |
Insoluble* |
| a:b:c =1.5618:1:1.2828 | Ksp=3.78E-07 | |||||
| CaSO4 |
Orthorhombic Dipyramidal |
6.991 |
6.996 | 6.238 | 3 |
Insoluble* |
| a:b:c =0.9992:1:0.8916 | Ksp=4.86E-04 |
|||||
| MgSO4 |
Monoclinic |
- |
- |
- |
260 |
- |
| - |
Ksp=4.67 | |||||
| Na2SO4 |
Orthorhombic Dipyramidal |
9.75 |
12.29 | 5.85 | 195 |
- |
| a:b:c =0.7933:1:0.4759 | Ksp=10.3 | |||||
| (NH4)2SO4 |
Orthorhombic Dipyramidal |
7.782 |
5.993 | 10.636 | 706 |
- |
| a:b:c =1.2985:1:1.7747 | Ksp=611 | |||||
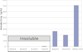
 Graphs:
Graphs:| Cell Dimensions: |
||
 |
||
| Solubility: |
||
 |
 |
 |
| Go
to |
|
 |
Conclusions |