
|
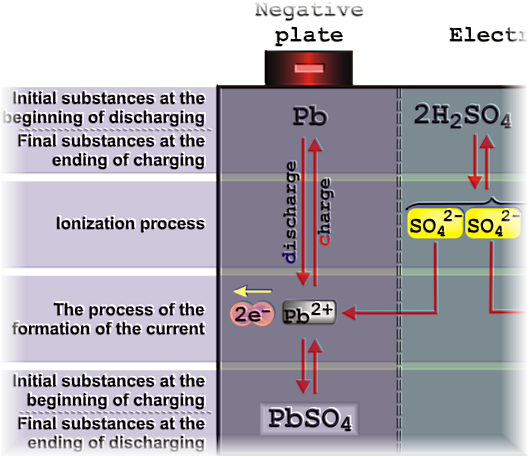
The reactions at the
negative plate lead at the formation (crystalization) of PbSO4
on the Pb substarte.
-
discharging - additives like BaSO4
or SrSO4
initate the cristalization of the PbSO4 in the oxidation phase
(discharging) the
-
charging - the PbSO4 must be
reduced completely from the surface of the electrode
|
|

|
The crystal structure of Ca sulfate is similar to
Pb
sulfate and CaSO4 on the lead substrate can provide seeds
for crystallizations
|

|
Bi
sulfate has a different crystal structure and
the surface of the crystal is not enough "active" to provide a seed for
PbSO4 crystals
|

|
Crystalohydrates like Eu2(SO4)3
or Eu2(SO4)3 can provide seeds for
crystallization of PbSO4 but more research is required
|

|
EuSO4 (Eu 2+) can be an
interesting field in the future.
|