|
 |
Discussions
|
|
 |
The oxidation process:
|
|
-
At the beginning the Eu was 3+
(Eu III sulfate octahydrate)
-
After heating at 300 °C, the water was
remove from the crystal.
-
At the beginning of the experiment we had Eu2(SO4)3
powder without water
-
In the oxidation phase the Eu2(SO4)3
powder tend to form again the hydrate sulfate and an oxide was form on
the surface of the electrode and passivate the surface.
|
|
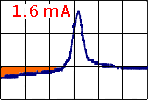
-
The current was very small in the oxidation
phase => an oxide
cover the surface and no PbSO4
crystals was formed on the surface (in the observation area).
|
 |
 |
The reduction process:
|
|
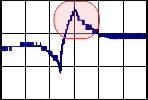
-
The peak in the reduction phase can be the
reduction of the Eu3+ to Eu2+
-
Eu (III) has the highest standard redox
potential, whitch makes possible its selective reduction [1]:
|
|
-
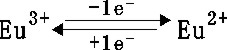
-
The chemical properties of E(II) are similar
to
those of the alkaline earth ions [2]
|
 |
 |
Possible candidate
|
|
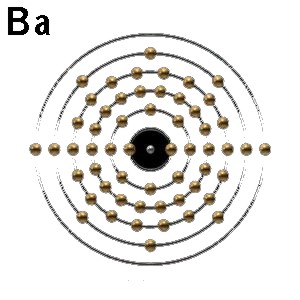
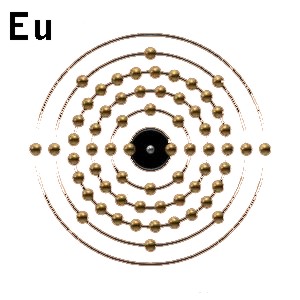
The electronic configuration of Eu (63) is
similar to Ba (56)
|
|
[Xe].6s2
|
[Xe].4f7.6s2
|
|
|
|
|
|
|
|
-
EuSO4 is insoluble in water
-
Difficult to obtain
-
Expensive
|
[1]
|
T. Hirato, H. Kajiyama, H. Majima, Y. Awakura, Electrolytic
reduction of Eu (III) to Eu (II) in acidic chloride solutions with
titanium cathode. Metall. Mater. Trans. B 26B (1995) 1175-1181.
|
[2]
|
T. Donohue, Photochemical separation of europium from
lanthanide mixtures in aqueos solutions. J. Chem. Phys. 67 (11) (1997)
5402-5404.
|
|
|